Goodbye eQMS,
hello iQMS !
Qity iQMS is not just an eQMS, but a complete
ecosystem for Medical Device Development.
Qity iQMS embeds a Quality Management System
in the tools you use every day to develop your product
and organize your work, effectively minimizing the paper work
and regulatory burden.
Strengths

Familiar tools, customised for compliance
Built on Jira, Confluence, and Bitbucket (used by 170,000+ companies), enriched with Qity's innovative apps, templates, automation, specifically configured for regulatory compliance.

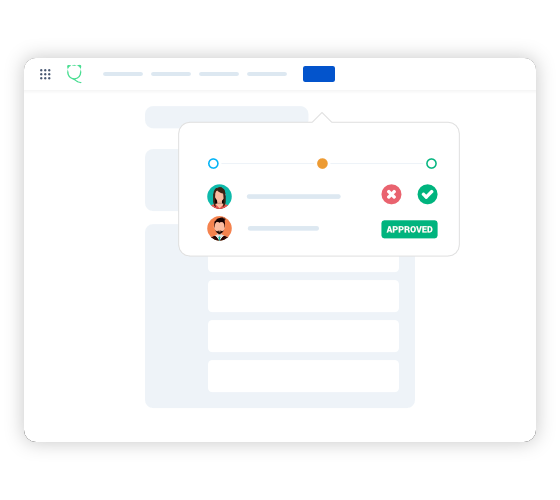
Document Control in Confluence and Jira
Say goodbye to importing/exporting documents into a separate Document Control System!
Qity's compliant Document Control System is exactly where it is supposed to be: the space where you write, collaborate and review your documents.

Grows with your organization
Qity's modular iQMS is tailored to the specific needs of your company, ensuring the right level of compliance at each stage of your product development.
Select the subscription plan tailored to your organization's needs.

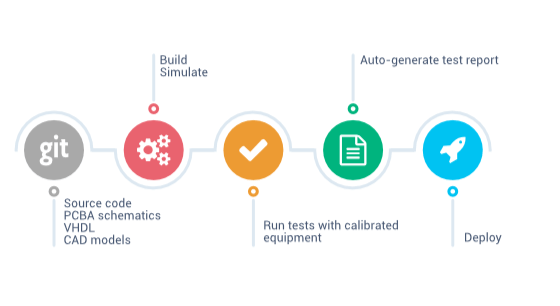
Automation, the future of regulatory product development
Dramatically reduce turn-around time of quality activities, by automating software and hardware tests with calibrated equipment, and testing your designs using modelling and simulation tools. Documentation (e.g. test reports) is automatically generated in the Quality Management System.

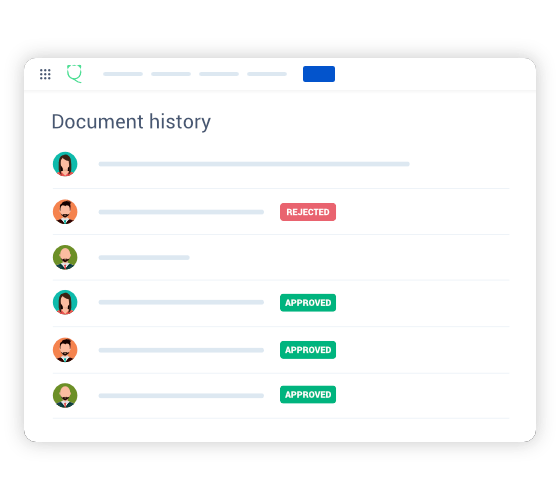
Complete traceability and audit trail
Unparalleled control and traceability, offering comprehensive compliant audit trail, full change tracking, detailed review commentary, for ultimate accountability and continuous audit-readiness.
iQMS Bundles
Concept Bundle
Develop a proof of concept

Core
Document Control
Design Control
Product Lifecycle
Risk
Regulations & Standards
Usability
Governance
Realization Bundle
Design your product

Everything in Concept Bundle
+
Purchasing
Equipment & Tools
Supplier
Facility & Infrastructure
Competence & Training
Bill of Materials
Pilot Bundle
Manufacture for compliance testing

Everything in Realization Bundle
+
Change Control
Audit
CAPA
Nonconformity
Deviation
Derogation
Inventory
Manufacturing
Commercial Bundle
Launch your product

Everything in Pilot Bundle
+
Market Surveillance
Order Processing
Customer Service
Submission

Global Atlassian Partner
As a global Atlassian Partner, we are qualified to set up and maintain your platform.
We stay on top of the latest developments from Atlassian and evaluate everything in-house before deploying to a customer.
Frequently Asked Questions
Is Qity iQMS validated?
Is Qity iQMS GDPR compliant?
Is Qity iQMS FDA 21 CFR Part 11 compliant?
Is customer support included in the monthly license fee?