
Lending a Hand to Life Science
Accelerate your project with Qity's smart tools and expert services.
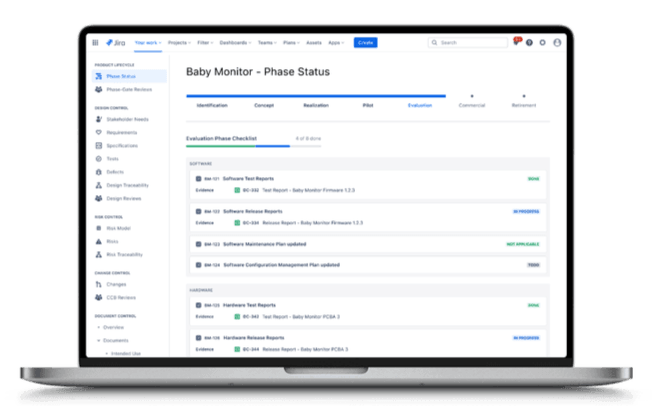
Game-Changing Quality System for Atlassian
Your quality management system shouldn't be a separate tool. Qity's iQMS centralizes all your QMS content—from CAPA's and Nonconformities to Change Controls—directly within Jira.

One Platform, Total Control
A single, integrated platform provides ultimate traceability and total control. Your team can connect quality events directly to their work and design tools, eliminating data silos and boosting efficiency.

From Disconnected Word Files to a Dynamic Quality System
Qity’s Document Control module turns Confluence pages into compliant, controlled documents. By integrating directly with your Jira-based QMS, it breaks down silos and automates workflows, so your team can work smarter, not harder.

Smart Automation
Say goodbye to manual, repetitive tasks. The Qity iQMS uses intelligent automation to streamline your quality events, eliminating human error and freeing up your team's time.

Paperless Manufacturing
Bridge the gap between your quality system and the factory floor with our Manufacturing Execution System (MES). The Qity MES integrates directly with your iQMS, allowing you to automate work instructions and seamlessly manage production records.
Expert Services
From quality assurance to clinical studies, our experts provide the strategic guidance and support you need to ensure compliance and successfully bring your product to market. We work with clients across the biotech, medical devices, digital health, and pharma sectors.
Compliance Testing
Electrical safety testing and troubleshooting in compliance with IEC 60601, IEC 61010 or non-medical standards.

Design & Development
Fast-track your medical device development through pre-tested, medical grade software and hardware building blocks.
Quality & Regulatory Affairs
Quality Management System, audits, device classification, applicable standards and regulatory strategy.

Security & Data Privacy
Embed cybersecurity and data privacy into your product development lifecycle, in compliance with MDCG 2019-16 and ISO-2700x.

Usability Engineering
From ideation, all the way to summative evaluation, in compliance with IEC-62366
AI Governance
Design, monitor, and certify AI systems aligned with ISO/IEC 42001 and the EU AI Act, embedding trust, traceability, and compliance from day one.
Boost Your Team's Skills
Equip your team with the essential knowledge and skills for successful product development.
We offer comprehensive training on medical product development, covering everything from design and usability to clinical research and validation.

Ready to accelerate?
Let's discuss how we can boost efficiency, de-risk your project and reduce time-to-market.













